Tin is a highly workable metal that was once as valuable as silver for jewellery, coins, and particular dishware. Today it is used in sheets for building and roof construction, soldering or joining metal parts, storage containers, and in alloys like Bronze and Babbitt metal.

Image source: https://search.creativecommons.org/photos/2ffee87f-78ae-449f-8d42-1cc9a9d34d78 by AJC1
What is Tin?
Tin (Sn) is a relatively soft and ductile metal with a silvery-white color. It has a 7.29 grams per cubic centimeter density, a 231.88 °C (449.38 °F) low melting point, and a 2,625 °C (4,757 °F) high boiling point. Tin is allotropic: it takes on more than one form. The general form is white tin, or beta tin, which presents a body-centered tetragonal crystal structure. The second allotrope, grey or alpha tin, possesses a face-centered cubic structure. Tin finds industrial application both as a metal and in chemical compounds. As a metal, it is employed in a wide variety of industrial applications, almost always in combination with other elements as an alloy or coating, since its intrinsic softness precludes its use as a structural material. Although tin is usually a minor constituent in alloys, it is an essential one due to its special properties confer improvements to the matrix metal.
The major tin commercial applications are tinplate, solder alloys, bearing metals, tin and alloy coatings (both plated and hot-coated), pewter, bronzes, and fusible alloys. In its chemical reactions, tin is found in two valence states (II and IV) and is amphoteric (capable of reacting as both acid and base). Also, it can link directly with carbon to form organometallic compounds. These properties have given rise to many important uses for tin chemicals; for example, in electroplating, agricultural and pharmaceutical products, plastics, and ceramics.
When and where was tin discovered?

Image source: https://search.creativecommons.org/photos/662249e6-e7c3-4d5d-af32-948b921a2c49 by Gary Lee Todd, Ph.D.
Tin, its alloys, and its compounds have been known to humans for thousands of years. In the Bible can be found several references to the element. Tin was known to other civilizations also. For example, the holy Hindu book Rig Veda, composed around 1000 B.C., mentions tin and other metals.
Bronze, a tin alloy, was created even earlier the pure metal. The mixture has properties that are different from any of the metals alone. The Egyptians, Mesopotamians, Babylonians, and Peruvians were making bronze approximately in 2000 B.C. Probably, when copper and tin compounds were heated together, the alloy was discovered accidentally.

Image source: https://search.creativecommons.org/photos/a7d2a2e8-a8d5-4b41-afea-82163d3fe4f5
Before the discovery of bronze, a lot of metal objects were made out of copper. But copper is soft and bends easily. The bronze is a much better replacement for tools, eating utensils, and weapons. Bronze marked significant progress in human civilization. This alloy advanced transportation methods, food making, and quality of life during the Bronze Age (4000—3000 B.C. ).
The origin of the name Tin is unknown. Some scholars believe that it originates from the Etruscan god Tinia. During the Middle Ages, tin was called by its Latin name, Stannum. From this name derives the element symbol, Sn.
Beyond bronze, the most important tin contribution to humankind was probably the humble tin can. The can has its origins in the perennial problem of how to feed an on-the-move army. Napoleon Bonaparte offered a reward in 1795 to anyone who could find out how to preserve food for military use. In 1810, Nicolas Appert, a French chef, won the 12,000-franc prize with his invention of canning: the process of sealing food or drink in a jar or bottle with the use of boiling water.
This discovery opened the way for the tin can invention just a year later. In 1810, British salesman Peter Durand got a license for using tinplated steel to can food.
The tin can appeared in America in 1818, and Thomas Kensett & Co, a manufacturing company, patented the tin can in 1825. The Civil War helped the increased fame of the tin can, as generals once again searched for a way to feed their soldiers.
Tin’s blooming days ended in the mid-20th century when Coors Brewery introduced the first aluminum can. Cheaper, lighter, and recyclable, aluminum rapidly overtook tin and steel.

Image source: https://search.creativecommons.org/photos/c84ec87a-6adb-4bdd-86be-7649de01d0d3 by Klearchos Kapoutsis

Image source: https://search.creativecommons.org/photos/67ded8d8-ead2-4d31-b532-2be989631d55 by Hamed Saber
What are its characteristics?
- Strength: Tin is one of the weakest metals. This property does not allow tin to be used on its own as a structural metal.
- Ductility: Tin is a very ductile metal at room temperature, and is also quite malleable. When chilled below 55 degrees Fahrenheit, tin slowly changes from a form known as “beta tin” to “alpha tin,” which is much less ductile. Tin is also much less ductile above roughly 392 degrees Fahrenheit.
- Conductivity: Tin and some of its alloys are excellent electrical conductors. Over half of the tin used industrially ends up in solder for making electrical connections.

Image source: https://search.creativecommons.org/photos/e0cd1733-9710-4cc3-b082-16ff8f235c4f by karen horton
One of the tin’s most interesting properties is its tendency to give off a strange screeching noise when it is bent. This sound is sometimes known as “tin cry”. It does not rust, corrode, or react at room temperature neither with oxygen or water. One of its major uses: as a coating protector of other metals. At higher temperatures, the metal reacts with both water (as steam) and oxygen to produce tin oxide.
Similarly, tin is attacked only slowly by dilute acids such as hydrochloric acid (HCl) and sulfuric acid (H 2 SO 4 ). Dilute acids are mixtures that contain small amounts of acid dissolved in large amounts of water. This property also makes tin a good protective covering. It does not react with acids as rapidly as do many other kinds of metals, such as iron, and can be used, therefore, as a covering for those metals.
In concentrated acids, tin dissolves easily, like in hot alkaline solutions, such as hot, concentrated potassium hydroxide (KOH). The metal also reacts with the halogens to form aggregates such as tin chloride and tin bromide. It also forms compositions with sulfur, selenium, and tellurium.
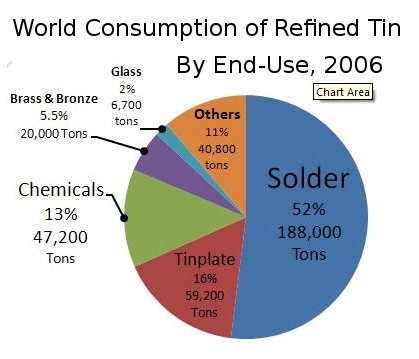
Image source: https://en.wikipedia.org/wiki/Tin#/media/File:TinConsChart.jpg
what are the various stages of metal processing?

Image source: https://search.creativecommons.org/photos/f2509034-638d-4c3e-8db0-ea5dfe4c8b75 by Alan Hunt
The tin extracting process depends on the ore deposit source and the impurity amount found in the ore. The Bolivian and English tin deposits are located deep underground and require using tunnels to reach the ore. In these deposits, the mineral may contain about 0.8-1.0% tin by weight. Malaysian, Indonesian, and Thai tin deposits are located in the gravel along streambeds and require dredges or pumps to reach the ore. The mineral in these deposits may contain as little as 0.015% tin by weight. Over 80% of the world’s tin is found in these low-grade gravel deposits.
Despite the source, each process consists of many steps during which the unwanted elements are physically or chemically removed, and also the amount of tin is progressively increased. A number of these actions are conducted at the mine site, while others are managed at separate facilities.

Image source: http://www.madehow.com/Volume-4/Tin.html
- When the gravel deposits are discovered, at or below the water level, within the stream; they’re flagged by a floating dredge operating in an artificial pond created along the streambed. The dredge digs the gravel employing a long boom fitted either with chain-driven buckets or with a submersed rotating cutter head and suction pipe. The gravel passes through a series of revolving screens and shaker tables onboard the dredge to separate the soil, sand, and stones from the tin ore. The residual mineral is then gathered and transported ashore for additional processing. When the gravel deposits are located in dry areas at or above the water level within the stream, they’re first broken with jets of water pumped through large nozzles. The resulting mud is captured in an artificial pond. A pump located at the rock bottom point within the pond pumps the slurry up into a wooden trough, called a palong, which encompasses a gentle downward slope along its length. The tin mineral, which is heavier than the sand and soil within the mud, tends to sink and is caught behind a group of wooden slats, called riffles. Periodically the trapped ore is dumped from the palong and is collected for further processing.
- The mineral enters the purification or dressing shed adjacent to the mining process. First, it passes through several vibrating screens to separate coarser foreign materials. It may then pass through a classifying tank filled with water, where the mineral sinks to the bottom while the smaller sediment particles are carried away. It may also pass through a floatation tank, where certain chemicals make the tin particles rise to the surface and overflow into troughs.
- Finally, the mineral is dried, selected again, and passed through a magnetic separator to remove any iron particles. The resulting tin concentrate is now about 70-77% tin by weight and consists of essentially pure cassiterite.
- The tin concentrate is placed in a furnace with carbon in the form of coal or fuel oil. If a tin concentrate has excess impurities, limestone and sand may are added to react with them. As the materials are heated to about carbon, the carbon reacts with the carbon dioxide in the furnace atmosphere to form carbon monoxide. The carbon monoxide reacts with the cassiterite in the tin concentrate to form crude tin and carbon dioxide. Limestone and sand react with any silica or iron present in the concentrate to form a slag.
- Because tin readily forms compounds with many materials, it often reacts with the slag. As a result, the slag from the first furnace contains an appreciable amount of tin and must be processed further before it is discarded. The slag is heated in a second furnace along with additional carbon, scrap iron, and limestone. As before, crude tin is created and collected along a certain amount of remaining slag.
- The residual slag from the second furnace is heated again to recover any tin that has formed compounds with iron. This material is known as the hard head. The remaining slag is discarded.
- After the first round in the furnace, the crude tin is placed in a low-temperature furnace along with the crude tin recovered from the slag. Because tin has a melting temperature much lower than most metals, it is possible to carefully raise the temperature of the furnace so that only the tin melts, leaving any other metals as solids. The melted tin goes down a slide and is collected in a poling kettle, while the other substances remain behind. This process is called liquidation and it effectively removes much of the iron, arsenic, copper, and antimony that may be present.
- The molten tin in the poling kettle is agitated with steam, compressed air, or poles of greenwood. This process is called boiling. The greenwood, being moist, produces steam along with the mechanical stirring of the poles. It was from this crude but effective use of wood poles that the poling kettle got its name. Most of the remaining impurities rise to the surface to form a scum, which is removed. The refined tin is now about 99.8% pure.
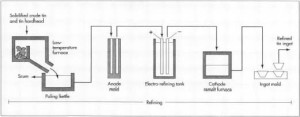
Image source: http://www.madehow.com/
How is it used today?
Tin is employed in the production of other alloys. Bronze, for example, is a tin and copper alloy. It is used in many industrial products, such as spark-resistant tools, springs, wires, electrical devices, water gauges, and valves.
One employment of tin was the manufacture of “tin foil”, a very thin sheet of tin used to wrap products. The tin protected the products from spoiling by exposure to air. Today, most tin foil is thin sheets of aluminum because aluminum is less expensive.

Image source: https://search.creativecommons.org/photos/bafae06c-a442-41dc-8129-c5db22c41e7b by Podknox
A very important application of tin is tinplating: the process by which a thin coat of tin is placed on the surface of steel, iron, or another metal as a protective layer. Perhaps the best-known example of tin plating is in the production of food cans. Tin cans are made of steel and are covered with a thin layer of tin. Today, most food and beverage cans are made out of aluminum.
Another tin alloy is Babbitt metal. Babbitt metal is a soft alloy made of any number of metals, including arsenic, cadmium, lead, or tin. Babbitt metal is produced to make ball bearings for large industrial machinery. The Babbitt metal is laid down as a thin coating on heavier metal, such as iron or steel since it retains a thin layer of lubricating oil more efficiently than iron or steel.
In the United States, the largest quantity of tin used goes to the production of solder. Solder is a combination of tin and lead, with a low melting point. Its employment is in connecting two metals. For example, metal wires are attached to electrical devices through solder. Solder is also used by plumbers, to seal the joint between two metal pipes.
Other uses are:
-
- tin chloride (SnCl 2 ): applied in the manufacture of colorants, polymers, and textiles; in mirror silvering; as a food preservative; as an additive in soap scents; as an anti-gumming agent in lubricating oils.
- tin oxide (SnO 2 ): adopted in the production of particular varieties of glass, ceramic glazes and colors, fragrances and cosmetics, textiles; and as a polishing substance for steel and other materials.
- tin chromate (SnCrO 4 or Sn(CrO 4 ) 2 ): brown or yellowish-brown mixtures employed as a coloring agent for porcelain.
- tin fluoride (SnF 2 ) and tin pyrophosphate (Sne 2 P 2 O 7): applied as toothpaste additive as protection against cavities.
Info sources:
https://www.britannica.com/technology/tin-processing
https://www.thebalance.com/metal-profile-tin-2340157
http://www.livescience.com/37355-tin.html
http://www.chemistryexplained.com/elements/T-Z/Tin.html
http://www.madehow.com/
